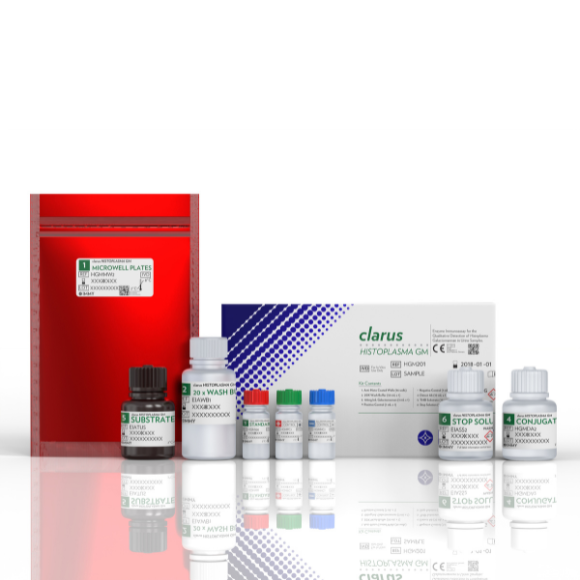
HISTOPLASMA GM ENZYME IMMUNOASSAY
The Histoplasma Galactomannan EIA offers an in-house assay to assist in reducing the number of misdiagnosed patients.
This test is a commercially available diagnostic kit for the detection of Histoplasma antigen. In-house rapid testing is much more cost effective than send-out testing.1
Specimen Types & Registrations
- CE: Urine
- FDA: Urine
Product Reference & Storage Conditions
- HGM201
- 2-8° c
Features & Benefits
Results In 2 HOURS, 15 MINUTES
Reduce Send-Out Testing
96 Patients Per Plate
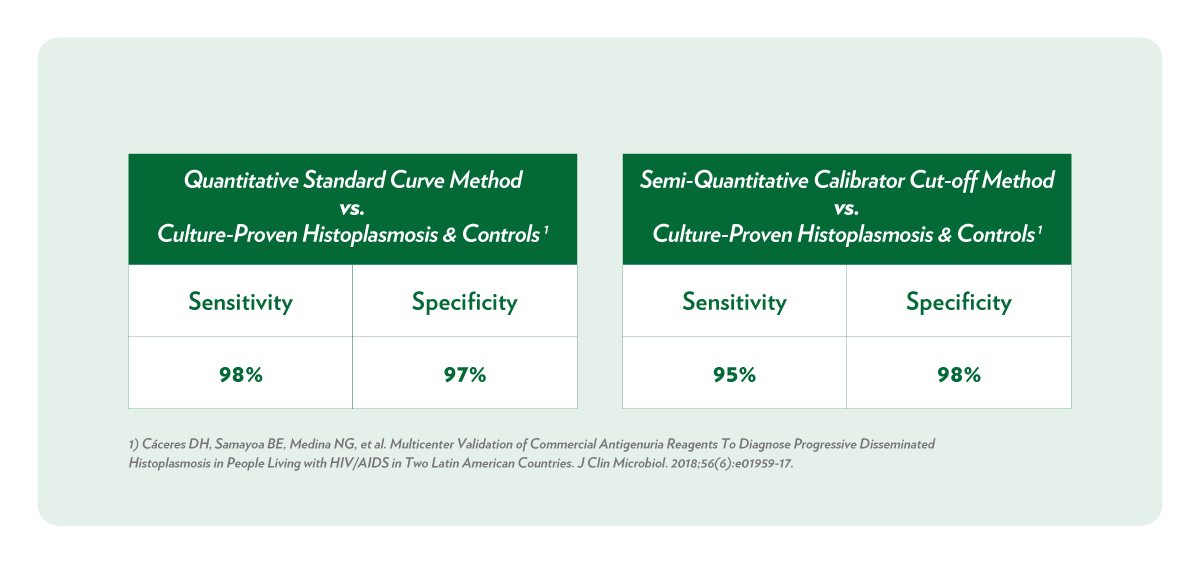
Clinical Relevancy
- DOCUMENTS
- STUDIES
Cáceres DH, Samayoa BE, Medina NG, Tobón AM, Guzmán BJ, Mercado D, Restrepo A, Chiller T, Arathoon EE, Gómez BL. Multicenter Validation of Commercial Antigenuria Reagents To Diagnose Progressive Disseminated Histoplasmosis in People Living with HIV/AIDS in Two Latin American Countries. J Clin Microbiol. 2018 May 25;56(6).
Falci DR, Monteiro AA, Braz Caurio CF, et al. Histoplasmosis, An Underdiagnosed Disease Affecting People Living With HIV/AIDS in Brazil: Results of a Multicenter Prospective Cohort Study Using Both Classical Mycology Tests and Histoplasma Urine Antigen Detection. Open Forum Infect Dis. 2019;6(4).
- ORDERING INFORMATION
Histoplasma Galactomannan EIA
96 tests
Ref: HGM201
|
Cáceres DH, Samayoa BE, Medina NG, Tobón AM, Guzmán BJ, Mercado D, Restrepo A, Chiller T, Arathoon EE, Gómez BL. Multicenter Validation of Commercial Antigenuria Reagents To Diagnose Progressive Disseminated Histoplasmosis in People Living with HIV/AIDS in Two Latin American Countries. J Clin Microbiol. 2018 May 25;56(6). |
|
Falci DR, Monteiro AA, Braz Caurio CF, et al. Histoplasmosis, An Underdiagnosed Disease Affecting People Living With HIV/AIDS in Brazil: Results of a Multicenter Prospective Cohort Study Using Both Classical Mycology Tests and Histoplasma Urine Antigen Detection. Open Forum Infect Dis. 2019;6(4). |
Histoplasma Galactomannan EIA |
96 tests |
Ref: HGM201 |
Discover the Life-Saving Potential
Reach out to learn how IMMY’s Histoplasma GM EIA can improve patient health with fast and accurate results.