COCCIDIOIDES Ab ENZYME IMMUNOASSAY
Coccidioidomycosis (Valley Fever) is difficult to diagnose due to lack of awareness and symptoms imitating many other respiratory infections. There were over 20,000 cases reported to the CDC in 2018. Valley Fever is said to cause nearly 30% of all community acquired pneumonia (CAP) cases in highly endemic areas.1
This assay offers two 96-microwell plates and two different antigen preparations, which greatly reduces the potential for error and enhances the sensitivity and specificity compared to competitive assays.
1) Valley Fever (Coccidiodomycoisis) Statistics. Updated 2022. https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html
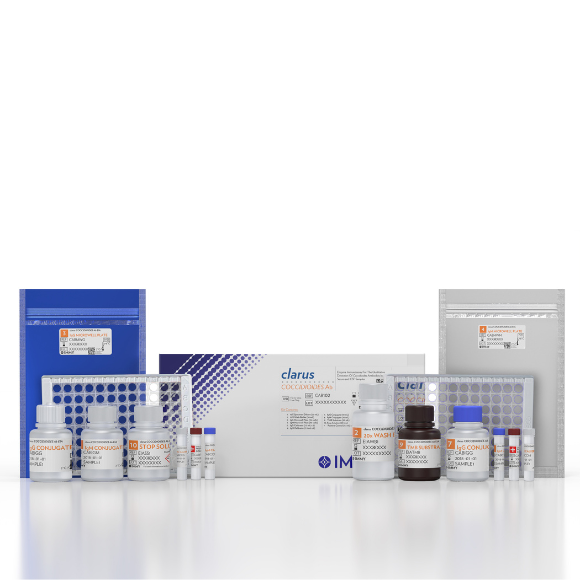
Specimen Types & Registrations
- FDA: Serum & CSF
Product Reference & Storage Conditions
- CAB102
- 2-30°c
Features & Benefits
Results in just 2 HOURS
Highly SENSITIVE & SPECIFIC
96 Well Plate
Clinical Relevancy Clinical Relevancy 2

- DOCUMENTS
- STUDIES
Comparative Study of Newer and Established Methods of Diagnosing Coccidioidal Meningitis
Stevens DA, Pappagianis D, McGuire M, et al. Comparative Study of Newer and Established Methods of Diagnosing Coccidioidal Meningitis. J. Fungi. 2020;6(3):125.
- ORDERING INFORMATION
Coccidiodes Antibody EIA
96 tests
Ref: CAB102
|
Comparative Study of Newer and Established Methods of Diagnosing Coccidioidal Meningitis Stevens DA, Pappagianis D, McGuire M, et al. Comparative Study of Newer and Established Methods of Diagnosing Coccidioidal Meningitis. J. Fungi. 2020;6(3):125. |
Coccidiodes Antibody EIA |
96 tests |
Ref: CAB102 |
Discover the Life-Saving Potential
Reach out to learn how IMMY’s Coccidioides Antibody EIA can improve patient health with fast and accurate results.