Clarus
HISTOPLASMA GM ENZYME IMMUNOASSAY
The Histoplasma Galactomannan EIA offers an in-house assay to assist in reducing the number of misdiagnosed patients.
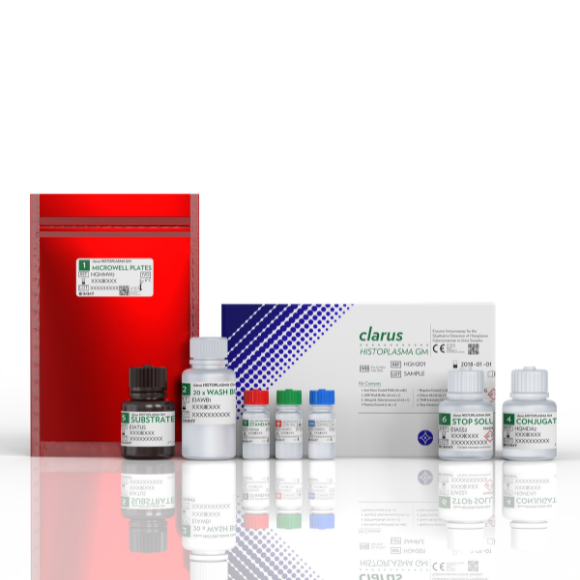
This test is a commercially available diagnostic kit for the detection of Histoplasma antigen. In-house rapid testing is much more cost effective than send-out testing.1
Specimen Types & Registrations
- CE: Urine
- FDA: Urine
Product Reference & Storage Conditions
- HGM201
- 2-8° c
Detect Early. Treat Fast. Save Lives.
Features & Benefits
Results In 2 HOURS, 15 MINUTES
Reduce Send-Out Testing
96 Patients Per Plate
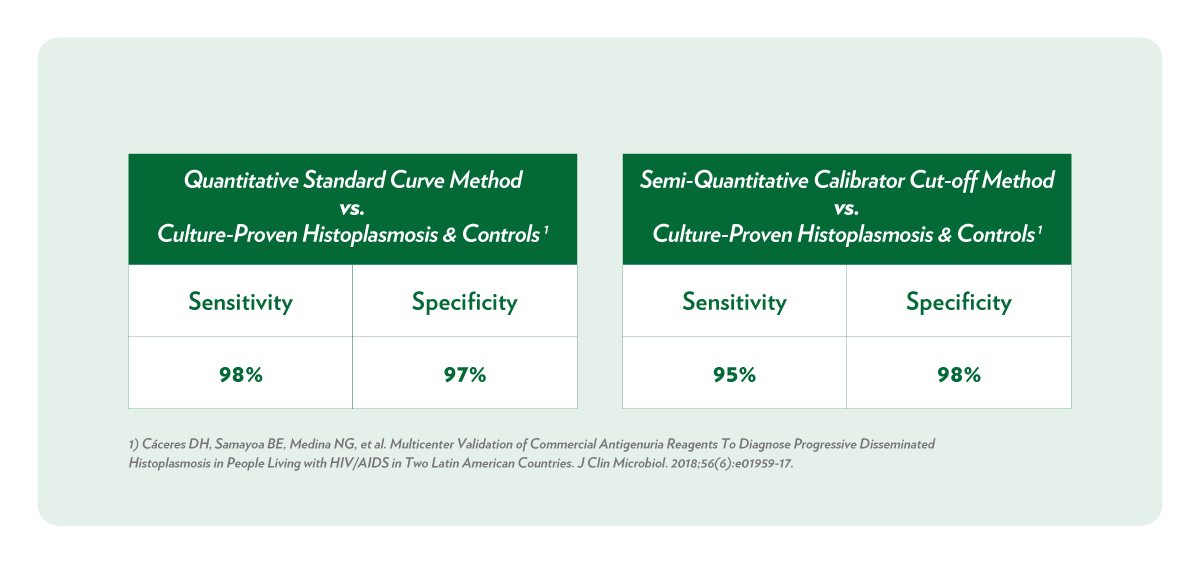
Clinical Relevancy
- DOCUMENTS
- STUDIES
- ORDERING INFORMATION
Histoplasma Galactomannan EIA
96 tests
Ref: HGM201
Histoplasma Galactomannan EIA |
96 tests |
Ref: HGM201 |
Discover the Life-Saving Potential
Reach out to learn how IMMY’s Histoplasma GM EIA can improve patient health with fast and accurate results.